Seretide user manual
Reed more and buy Seretide here
Seretide is a combined drug with bronchodilator, anti-inflammatory, anti-asthma, glucocorticoid, beta-adrenomimetic action.
Release form and composition
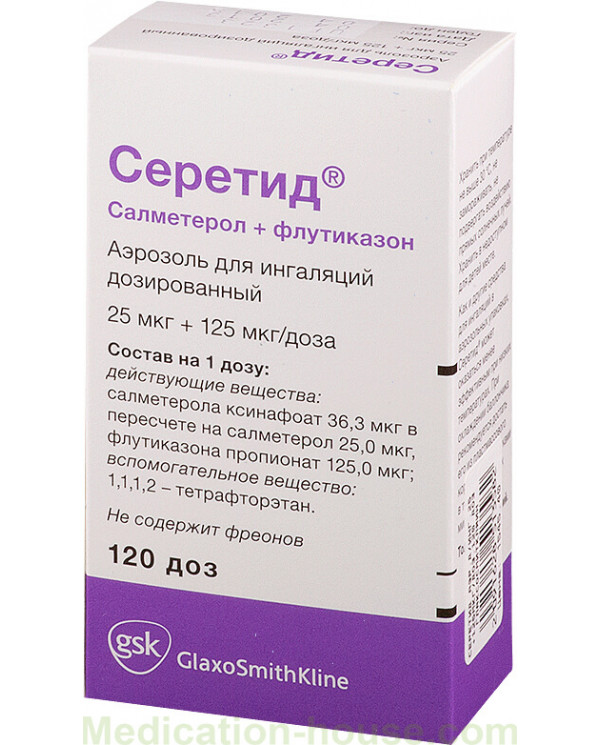
Dosage form - metered-dose aerosol for inhalation: suspension of white or almost white color (120 doses in an aluminum inhaler with a concave bottom, a hermetically closed metering valve, 1 inhaler in a cardboard pack without visible surface defects and the valve complete with a plastic nebulizer).
The content of active substances in 1 dose of Seretide:
salmeterol xinafoate: 0.0363 mg, equivalent to 0.025 mg salmeterol;
fluticasone propionate: 0.05 mg, 0.125 mg or 0.25 mg.
Auxiliary components: 1,1,1,2-tetrafluoroethane.
Indications for use
The regular use of Seretide is indicated for patients with bronchial asthma, the treatment of which involves the simultaneous use of long-acting beta2-adrenergic agonists and inhaled glucocorticosteroids (GCS):
patients in whom continuous monotherapy with inhaled GCS and periodic use of short-acting beta2-adrenergic agonists do not allow complete control of the disease;
patients with complete control of bronchial asthma during treatment with long-acting beta2-adrenergic agonist and inhaled corticosteroids;
patients with persistent bronchial asthma, who are shown to have GCS (in the form of initial maintenance therapy) to achieve control of the disease.
In addition, Seretide is used in the form of maintenance therapy for chronic obstructive pulmonary disease (COPD) with FEV1 (forced expiratory volume for the first second of forced expiratory maneuver) less than 60% of the set values (before inhalation of the bronchodilator) and history of repeated exacerbations in patients with persisting severe symptoms of the disease against the background of regular therapy with bronchodilators.
Contraindications
age up to 4 years;
individual intolerance to the components of Seretide.
Caution should be used with Seretide in acute or latent pulmonary tuberculosis, cataracts, glaucoma, thyrotoxicosis, osteoporosis, bacterial, fungal or viral infections of the respiratory system, in the treatment of any drugs of the sympathomimetic group (risk of increased systolic blood pressure (BP) and heart rate ( Heart rate), especially in patients exceeding therapeutic doses), with cardiovascular diseases, including arrhythmias (supraventricular tachycardia, extrasystole, fibrillation atrium, ventricular extrasystole), with hypokalemia, since elevated doses of sympathomimetics can cause a transient decrease in the concentration of potassium in the blood serum; in patients with diabetes.
During pregnancy and breastfeeding, the drug is prescribed only after assessing the ratio of benefit and risk of therapy, if the expected effect for the mother is much higher than the possible risk to the fetus and the baby.
Dosage and administration
Aerosol is used only as an inhalation.
The optimal effect is achieved with regular use of Seretide, even during the absence of clinical symptoms of the disease.
Before first use, check the quality of the inhaler. Having removed the cap from the mouthpiece, the inhaler is taken into the hand, putting the thumb on the base under the mouthpiece and holding with the other fingers, shake the inhaler intensively, releasing a couple of jets into the air. After making sure that the inhaler is working and has no visible damage, you can start using it.
Before each spraying, it is recommended to shake the contents of the can to mix evenly. Then the inhaler is held upright with the fingers of one hand upside down, so that the thumb is on the base under the mouthpiece and is brought to the mouth. The procedure should be performed calmly, without rushing. Having exhaled deeply (as far as possible), the mouthpiece is placed in the mouth between the teeth and the lips are tightly closed around it (without biting the mouthpiece). On inhalation through the mouth, pressing the bottom of the inhaler, spray Seretide, continuing to slowly, deeply inhale. After spraying, the inhaler is removed from the mouth, holding the breath. Breathing should be held as long as possible, then, after about 0.5 minutes, make a second spray. After the procedure, rinse your mouth well with water and spit it out. The inhaler should immediately be closed by pressing and snapping the cap onto the mouthpiece in the desired position without effort.
The first few procedures can be performed in front of the mirror. Particular attention should be paid to inspiration, it should be very slow and begin before pressing the valve directly.
If a foggy cloud appears from the corners of the mouth or the upper part of the inhaler, the procedure should be repeated.
Adults should help young children use the inhaler. For patients for whom the correct breathing maneuver is difficult (including young children), Seretide can be used through a spacer.
The appointment of a dosage regimen, course duration or dose change should be done by a doctor, regularly evaluating the effectiveness of therapy.
For the treatment of bronchial asthma, the lowest dose that provides effective control of symptoms should be used. It is recommended that one of the forms of Seretide be prescribed in which the dose of fluticasone propionate corresponds to the severity of the disease.
Patients in whom taking Seretide 2 times a day provides complete control over the symptoms can be assigned a minimum effective dose with a frequency of administration of 1 time per day.
The use of the drug can improve the control of symptoms of bronchial asthma in patients who do not achieve a sufficient therapeutic effect from taking inhaled corticosteroids. When replacing with Seretide, an equivalent dose of previously administered GCS should be used.
For patients in whom the course of bronchial asthma can only be controlled by inhaled GCS, switching to treatment with a combined aerosol can reduce the dose of GCS necessary to control the course of asthma.
Recommended dosage:
bronchial asthma: patients over 12 years old (at a dose of 0.025 mg of salmeterol and 0.05 mg or 0.125 mg, or 0.25 mg of fluticasone propionate) - 2 sprays 2 times a day; children 4 years and older (at a dose of 0.025 mg of salmeterol and 0.05 mg of fluticasone propionate) - 2 sprays 2 times a day;
COPD: adults (at a dose of 0.025 mg of salmeterol and 0.25 mg of fluticasone propionate) - no more than two inhalations 2 times a day.
In case of impaired renal and / or liver function, elderly patients do not need a dose reduction.
The inhaler requires careful care, it must be cleaned at least 1 time per week. Without removing the metal spray can from the casing, remove the protective cap from the mouthpiece and wipe it with a cotton swab from the inside and outside, and the plastic casing only from above. Then close the mouthpiece with the protective cap.
Side effects
Adverse events identified during clinical trials:
from the respiratory system, chest and mediastinal organs: often - hoarseness and / or dysphonia; infrequently - pharyngeal irritation;
parasitic and infectious pathologies: often - candidiasis of the mouth and throat, in patients with COPD - pneumonia;
from the nervous system: very often - headache; infrequently - tremor;
on the part of the heart: infrequently - tachycardia, palpitations, atrial fibrillation; rarely - arrhythmia, including supraventricular tachycardia, extrasystole and ventricular extrasystole;
from the immune system: infrequently - skin reactions of hypersensitivity, shortness of breath; rarely anaphylactic reactions;
on the part of nutrition and metabolism: infrequently - hyperglycemia; very rarely - hypokalemia;
from the endocrine system: infrequently - cataract; rarely - glaucoma;
mental disorders: infrequently - sleep disturbances, anxiety; rarely - changes in behavior, including irritability, hyperactivity (more often in children);
from the musculoskeletal system and connective tissue: often - arthralgia, muscle cramps;
dermatological reactions: infrequently - bruising.
Side effects of post-registration observations:
from the respiratory system, chest and mediastinal organs: rarely - paradoxical bronchospasm;
from the immune system: rarely (hypersensitivity reactions) - bronchospasm, angioedema (often swelling of the face, mouth and pharynx);
from the endocrine system: rarely (systemic effects) - cushingoid symptoms, Cushing's syndrome, decreased bone mineral density, inhibition of adrenal function, growth retardation in children and adolescents.
special instructions
Seretide is not intended to relieve acute attacks of bronchial asthma. For this purpose, the patient should always have with him a short-acting quick-acting inhalation bronchodilator (including salbutamol).
The increase in dyspnea after the direct use of an aerosol indicates the development of paradoxical bronchospasm. The patient must immediately apply a short-range inhaled bronchodilator, cancel Seretide and consult a doctor.
The drug can be used for initial maintenance therapy of persistent bronchial asthma only in patients with indications for the use of corticosteroids and after determining their approximate dose.
An increase in the frequency of use of short-acting bronchodilators indicates a worsening of the control of the disease, in which case you should consult a doctor.
An immediate appeal of the patient to the doctor is required in case of a sudden and increasing deterioration in the control of bronchospastic syndrome, in the absence of adequate control of the disease from the dose used.
Abrupt withdrawal of Seretide can cause an exacerbation of the condition in patients with asthma, and with COPD it can cause decompensation symptoms, so discontinuation of treatment should be carried out under the supervision of a doctor, gradually reducing the dose.
The doctor should consider the possibility of developing pneumonia with the use of Seretide in the treatment of COPD. It is necessary to carefully monitor the patient's condition due to the similarity of the symptoms of the two pathologies.
Because of the risk of developing unwanted systemic effects for the treatment of bronchial asthma, the lowest effective doses should be used to control the symptoms.
The patient should be aware of the need for the use of corticosteroids in emergency stressful situations, accompanied by inhibition of the adrenal glands.
During surgical operations and resuscitation, the degree of adrenal insufficiency should be determined.
With prolonged treatment of children with inhaled GCS, it is recommended to regularly measure their growth.
Particular caution and regular monitoring of adrenal cortex function is required in the treatment of patients transferred to fluticasone inhalation therapy with propionate from oral corticosteroids. In this case, systemic corticosteroids should be abolished gradually.
In patients with hypoxia and exacerbation of bronchial asthma, it is necessary to control the content of potassium ions in the plasma.
Metal spray can not be immersed in water.
Caution should be exercised when driving vehicles and mechanisms, since the effect of the drug can cause the development of side effects that affect the speed of psychomotor reactions.
Drug interaction
The use of non-selective and selective beta-blockers is indicated only in case of urgent need, because of the risk of developing bronchospasm, such combinations are recommended to be avoided in the treatment of patients with Seretide.
With the simultaneous use of Seretide:
monoamine oxidase inhibitors, tricyclic antidepressants - increase the risk of unwanted effects from the cardiovascular system;
xanthine derivatives, diuretics and corticosteroids - contribute to the development of hypokalemia, especially in patients with hypoxia, exacerbation of bronchial asthma.
Since inhalation of fluticasone propionate does not cause an increase in its concentration in plasma due to the high systemic clearance in the intestine and liver under the influence of the CYP3A4 isoenzyme of the cytochrome P450 system and intensive metabolism, the risk of developing a clinically significant interaction with other drugs is negligible.
Ritonavir (a highly active inhibitor of the CYP3A4 isoenzyme) can cause a sharp increase in the concentration of fluticasone propionate in the plasma, and as a result, a significant decrease in the content of serum cortisol. Intranasal or inhaled use of fluticasone propionate in combination with ritonavir can cause inhibition of adrenal function and Cushing's syndrome. Therefore, the simultaneous administration of these drugs should be avoided, except in cases where the risk of systemic side effects of GCS is inferior to the potential benefit of therapy for the patient.
Other inhibitors of the CYP3A4 isoenzyme do not cause a significant increase in the concentration of fluticasone propionate in the plasma and their intake practically does not affect the level of serum cortisol. Nevertheless, strong CYP3A4 inhibitors, such as ketoconazole, are recommended to be used with caution, since taking them increases the risk of increasing the systemic effects of Seretide.
The drug is compatible with cromoglicic acid.
Terms and conditions of storage
Keep out of the reach of children.
Store in a place protected from direct sunlight at temperatures up to 30 ° C, do not freeze.
Shelf life is 2 years.
Terms of sell
You can buy Seretide without a prescription.